A Deep Dive into the Structure of Hydrogen
Hydrogen gas holds pride of place as the first element of the periodic table, with the symbol ‘H’. It is the simplest, lightest, and smallest of the noble gases and has been shown to have a therapeutic effect on over 150 human diseases! But what is this all-important nano-molecule we know as Hydrogen? It consists of only one electron and one proton and, under normal conditions, it exists primarily in its diatomic form as molecular hydrogen (H2 gas). It is what powers the sun by fusion to produce helium. Hydrogen is the center of the prevailing cosmological model that describes the early development of the Universe as well as the origin of life itself!
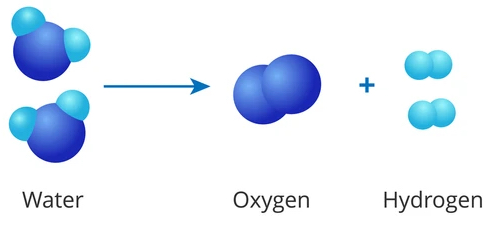
WATER: OXYGEN + HYDROGEN = LIFE
Interestingly, water, which is essential to life, is formed by the combination of oxygen (a powerful oxidizer and essential for life) and hydrogen (a powerful reducer and essential to life). It therefore makes perfect sense that molecular hydrogen has therapeutic potential.
MOLECULAR HYDROGEN

Molecular hydrogen gas, or H2 (g), is the primary form in which hydrogen is found. In other words, two hydrogen atoms (H) are covalently bonded (a type of chemical bond) together as H-H. Because there are two hydrogen atoms, we call this diatomic hydrogen (“di” meaning two). Because the hydrogen atoms are covalently bonded together, they form a molecule; so, H2 is also referred to as molecular hydrogen. We can also refer to it as dihydrogen. The hydrogen molecule contains two protons and two electrons making it a neutrally-charged molecule. It is a colourless, odourless, tasteless, non-metallic, highly flammable gas, and very explosive above a 4.6% concentration by volume. It is this form of hydrogen that has been shown to exert the wide range of therapeutic effects as set out in our Research Studies’ tab. Some articles refer to this as “hydrogen-rich water”. It is the smallest molecule in the universe, and this extremely small size and its high lipid solubility allows it to easily diffuse into the subcellular compartments of the mitochondria and other locations.
POSITIVELY-CHARGED HYDROGEN CATION: THE ‘H’ IN pH
A positively charged hydrogen ion (H+ cation) is also known as just a ‘proton’. Because a hydrogen atom only has one electron and one proton, if the atom loses its electron it’s only a proton. It is this form of hydrogen that drives the enzyme ATP synthase in the mitochondria. The mitochondria is considered the “powerhouse” of the cell as it produces the majority of the ATP (adenosine triphosphate), which is the energy currency of our cells.
The hydrogen ion (H+) is what is responsible for the pH of the water (i.e. acidic or alkaline). Water dissociates to form protons (H+) and hydroxides (OH–). That is: H2O => H+ + OH–. This is called the ‘self-ionisation’ of water.
Water: A Molecule of Life
THE IMPORTANCE OF WATER
Water is the universal solvent; it can exist as a solid, liquid or gas. It is necessary for the functions of life and for life to function. Water is the life-giving fluid that is always at the heart of creation. Its presence supports life, and its absence brings death. Water in liquid state is the principle criteria for planetary habitability.
The importance of water is well known. Obviously life would not exist without it. Indeed, water is virtually the most important nutrient for our health. You can go without food for about a month; however, going only three days without water can be fatal. It is used for maintaining body temperature, absorbing nutrients, eliminating waste, and many cellular processes including enzyme catalysis. The average adult is 55% to 75% water by weight; the variation is due to gender, height, lean body and shape. Someone with a 55% totally body water can be more hydrated than someone with a 65% total body water because it is relative to each individual based on their body type. Optimal body hydration is important for optimal cell hydration and cell function (i.e. communication, signaling, cell metabolism, gene expression, etc.). About 2/3 of the water in the body is intracellular and about 1/3 is extracellular.
HOW IMPORTANT IS HYDRATION?
It makes sense that maintaining optimal hydration is requisite for overall health and longevity. In fact, the 2003 heat wave in Europe led to an excess of 50,000 deaths. The majority of these deaths have been attributed primarily to dehydration. Indeed, dehydration is a primary cause for the hospitalization of the elderly and in many patients it often results in fatalities. Dehydration is also linked to infection and if overlooked, mortality may be greater than 50%. Did you know that losing only 1%-2% of your bodyweight in water significantly impairs both athletic and cognitive performance?
The hydrogen bonding of water molecules is also responsible for ionizing the water to form an hydroxide (OH-) and a hydronium ion (H3O+). 2H2O ? H3O+ + OH–. This makes water amphoteric; it can be an acid or a base.
As seen in the schematic below, a water molecule can pull hydrogen off another water molecule, which results in the two ionic species.
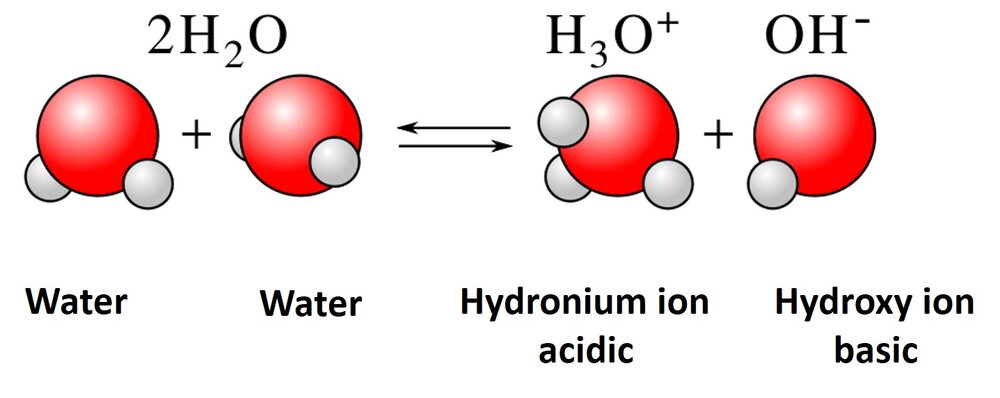
This is what is responsible for the pH of the water. pH means the negative logarithmic concentration of the H3O+ species.
Article by Today’s Practitioner
